The CO2 Conundrum
Ocean acidification, caused by the addition of huge amounts of carbon dioxide to the marine environment, is a problem that has come to the fore relatively recently. Jason Hall-Spencer, Professor of Marine Biology at Plymouth University, places it within the context of the myriad other threats that face the world’s oceans.
Our use of finite natural resources is accelerating. Coupled with our poor management of renewable resources, this means that the earth has entered a phase of mass extinction; biodiversity is being lost across the planet.
Since the 1950s, coastal ecosystems have been radically transformed by human activities. The oceans have fared no better. Within a generation, fishing vessels using fossil fuels have removed most large fish from ecosystems and caused continental shelf habitats to lose their diversity. Extensive damage is now also occurring all along the edges of continental shelves and even on remote sea mounts.
The good news is that governments are at last getting serious about cutting carbon dioxide (CO2) emissions and enforcing restrictions on destructive practices.
A changing planet
The past 60 years have without doubt seen the most profound transformation of our relationship with the natural world in the history of humankind. Since 1950, the human population has trebled (now at 7.3 billion, it is still rising fast); water use is up from 1,800 to 5,800 cubic kilometres per year; the number of rivers dammed has risen from 4,000 to 28,000; fertiliser consumption has jumped from 40 million to 280 million tonnes a year, quadrupling inputs of nitrogen to the coastal zone; and 65% of the atmospheric ozone has been lost. Motor vehicle use is up from 30 million to 750 million vehicles on the road and international tourism has really boomed, rising from fewer than one million arrivals per year in the 1950s to 600 million today.
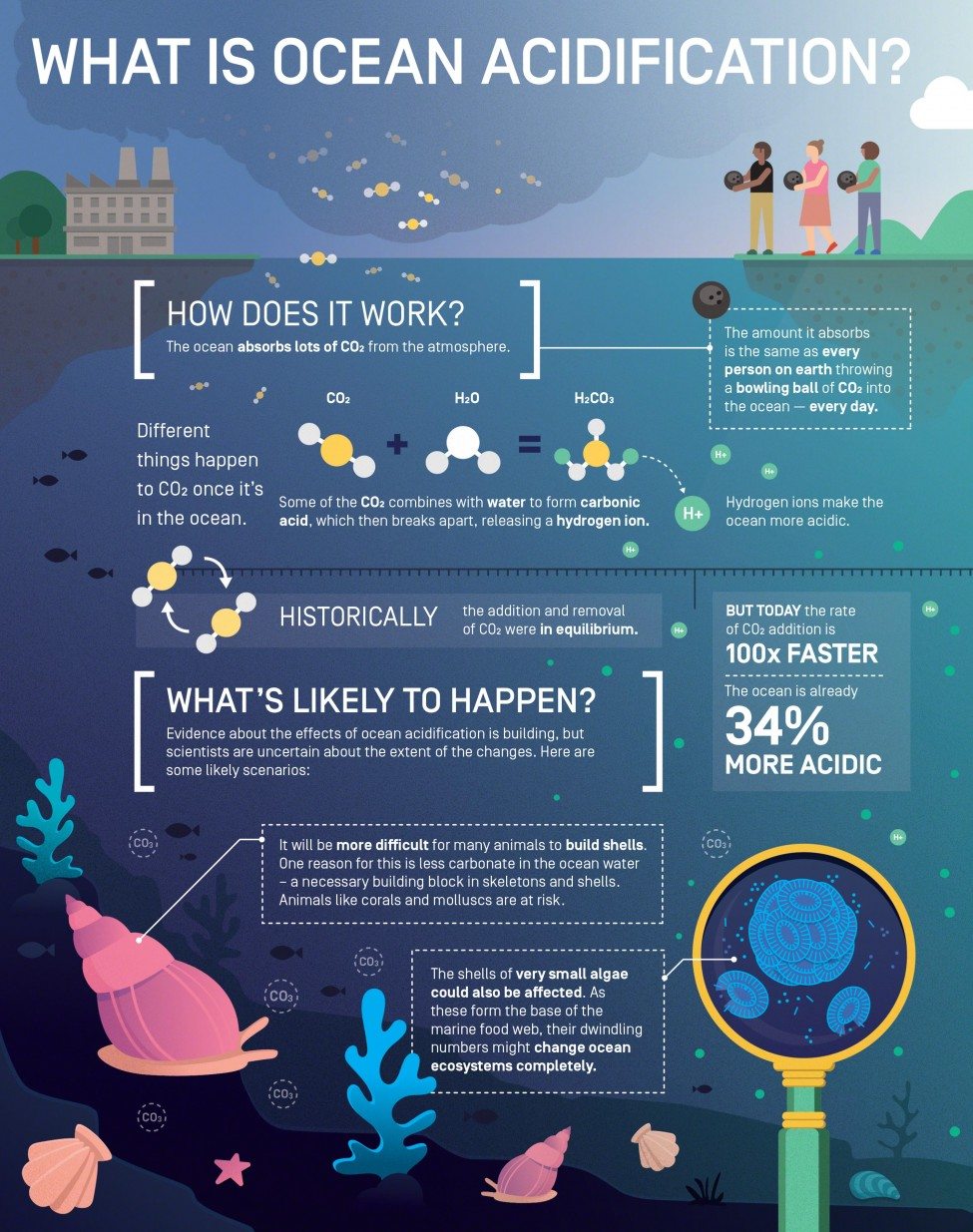
All this has led to a great acceleration in our use of the earth’s resources. Atmospheric methane and CO2 concentrations have increased, causing the temperature of surface sea water to rise. We know from ice-core data that these warming gases are at much higher levels than at any time in the past 800,000 years, an era that includes a sequence of glacial periods and warm periods. Now we have entered unknown territory, as the excess CO2 in the atmosphere is acidifying the oceans.
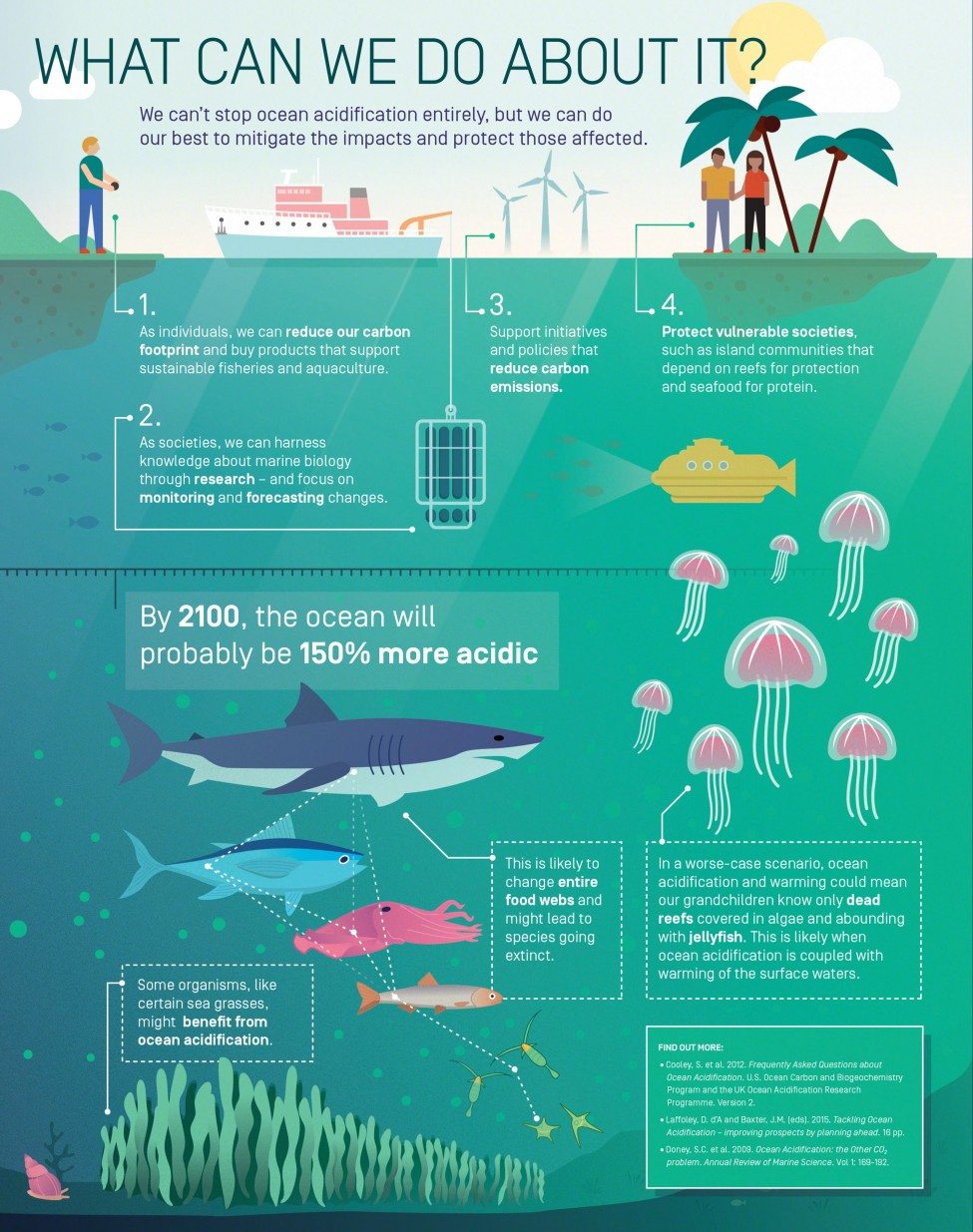
The monitoring of surface sea water off Hawaii and on both sides of the North Atlantic clearly shows increases in CO2 levels that are tracking atmospheric increases. Carbon dioxide forms carbonic acid when it dissolves in water and has caused a 34% increase in the acidity (i.e., the concentration of hydrogen ions, H+) of sea water since 1800; by 2100 it will have caused an increase of about a 150% in surface ocean acidity. This is the fastest rate of chemical ocean change for millions of years, and perhaps in all time, since the rate at which fossil fuels are being burnt is geologically unique. In effect, the amount of carbon taken up by the oceans at present equates to every person on earth throwing carbon of the weight of a bowling ball into the sea every day.
Clearly ocean acidification is not acting in isolation. Rising CO2 levels are also causing ocean warming, which is damaging tropical coral reefs, melting Arctic ice, thawing tundra and causing the distributions of many marine species to shift towards the poles. In low-latitude areas, warming waters are causing oxygen depletion, as warm water can’t hold as much oxygen as cold water can. Also at low latitudes, mid-ocean gyres with low productivity are expanding because increased thermal stratification suppresses mixing and so starves surface waters of the nutrients that underpin productivity in the food web.
Research into ocean acidification is the ‘new kid on the block’ among planetary environmental issues. As evidence rolls in from across the globe it is becoming clear that many organisms are likely to be affected because not only does ocean acidification increase the amount of carbon available for photosynthesis and so is a resource for primary production, but it also lowers the amount of carbonate in the water, so that it can become corrosive to exposed skeletons and shells.
The acidification of the oceans has myriad biological ramifications because the transport of materials across cell membranes is influenced by H+ concentrations and so this can affect reproduction, behaviour, respiration and growth. This is thought to explain why the fossil shells found after high-CO2 mass extinctions are dwarf forms, since smaller animals are better able to cope with the stress of ocean acidification.